Meta-analysis occurs when a review is carried out in a predetermined order (i.e., methodically) and the results are quantitatively assessed. Meta-analyses have become more widely published in recent years, with 1,473 titles published in 2007 and 176,704 in January 2020. Clinicians, researchers, and policymakers can benefit from well-designed and presented meta-analyses. Therefore, this article also serves as a guide for authors to learn about the important elements of meta-analyses while writing their reviews.
Subtypes of Study Design Described
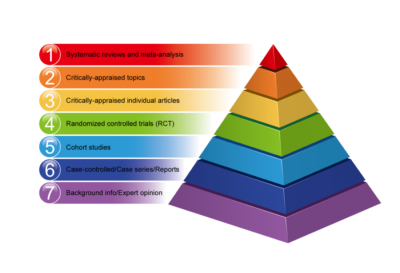
Traditional meta-analyses and unconventional meta-analyses are the two forms of meta-analyses that are most commonly classified. Traditional meta-analyses combine randomized controlled trials (RCTs), observational studies, diagnostic studies, and prognostic studies to analyze the effectiveness of one intervention vs another (e.g., an investigational intervention, usual practice, placebo) using aggregated data from previous studies.
Meta-analyses of RCTs are the most effective way to summarize the positive and negative impacts of therapies. RCTs, on the other hand, are not without bias, as poor randomization methods, a lack of blinding and inadequate outcome data can all lead to high degrees of bias. Because cohorts and case-control studies are designed differently, meta-analyses of observational research have greater obstacles; yet, meta-analyses frequently integrate both. In observational studies, there are several kinds of bias; one of the most common is selection bias, which occurs when groups of interventions being examined are not comparable at baseline. As a result, the calculated impacts of interventions may be related to other causes (eg, confounding by indication, where sicker patients may receive more aggressive or newer treatments than those in healthier patients).
Diagnostic test accuracy or meta-analyses of diagnostic studies investigations of people at risk of developing an illness, meta-analyses compare specific tests to a gold standard (ie, there are patients with and without disease). Current approaches construct summary areas under the receiving-operating characteristic curves after accounting for the natural correlation between sensitivity and specificity. Meta-analyses of prognostic studies compute a summary effect of a specific factor’s correlation with an outcome or a summary effect of predicted accuracy (overall performance, calibration, and discrimination).
Network meta-analysis (NMA), individual patient data (IPD) meta-analysis, and unusual event meta-analysis are all nontraditional meta-analyses that primarily use data from RCTs. An NMA compares numerous interventions at once, combining direct (i.e., actual comparisons) and indirect (i.e., computed from other comparisons) impacts; some comparisons summarize both direct and indirect effects. Because access to individual-level data is more difficult, IPD meta-analyses are uncommon. They use data from every individual patient and allow specific subpopulations to be analyzed in greater detail than is feasible with aggregated data. In rare event meta-analyses, outcomes that correspond to less than 10% of the total number of people in a trial arm are evaluated. A meta-analysis of rare events indicated that these analyses must utilize extremely particular approaches due to the paucity of occurrences and poor reporting of damaging events.
Advantages of Study Design
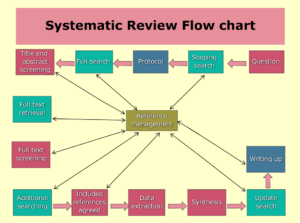
If done correctly, a systematic review will uncover all available material for a specific research issue and assess the body of evidence’s quality. The findings of subsequent meta-analyses may have an impact on clinical practice. It is critical to work with an information expert or librarian to build effective search tactics. This usually entails using at least three search engines as well as RCT repositories. It’s also critical that at least two researchers pick studies independently, extract study and outcome data, and assess the possibility of bias, with any disputes handled by a third researcher.
Meta-analyses have the advantage of having more power to identify an effect of an intervention on an outcome than individual trials. Pooled effect CIs can be more precise (i.e. narrower) than the CIs from most individual research. Researchers can also use meta-analyses to see how studies differ in terms of populations, interventions, controls, outcomes, intervention time or follow-up, or design (i.e. methodologic heterogeneity) and how different interventions’ effects are among studies (ie, statistical heterogeneity).In meta-analyses, effects across subgroups of individuals within trials are also feasible; subgroups are determined by patient baseline characteristics (eg, age, sex, severity of disease, diabetes). When IPD is available, it is possible to control for confounders, but mainly for exploratory reasons to establish new ideas for future investigations. Systematic reviews can also be used to assess the quality of the evidence for a specific research question. Importantly, evidence quality is assessed per outcome rather than per study.
Study Design’s Drawbacks
If they aren’t preceded by a thorough, systematic review, meta-analyses can be misleading. When heterogeneity is high and its sources have not been adequately explored and addressed, combining many studies with methodologic differences and varied impacts on results might be troublesome (eg, adjustment for confounders, exclusion of heterogeneous studies). When methodologic and statistical heterogeneity is substantial, authors should explain why they chose to conduct meta-analyses; in most circumstances, we do not recommend conducting meta-analyses in the context of high heterogeneity. It’s also debatable if summarizing enormous volumes of data with a single number is a good idea.
A meta-analysis with a low risk of bias will not be produced by combining many studies with a high risk of bias. The quality of individual research being examined and analyzed determines the strength of pooled estimates. There is no way to account for the possibility of bias in individual research; one strategy is to exclude those trials out of primary analyses or secondary sensitivity analyses. To combine studies in a meta-analysis, you’ll need to assess the quantity and sources of heterogeneity, explain how to deal with it, and have the right theoretical knowledge and training. A meta-analysis is more than just entering numbers into software to get pooled effects.
We do not propose merging multiple designs to get a pooled effect estimate; instead, stratifying by research design is recommended. When authors combine RCTs with observational studies, for example, it is best to calculate their effects separately. The problem with observational studies evaluating interventions is that they are not comparable, and the intervention’s effect can be explained by other variables or predictors of the desired outcome. Because observational studies have distinct sources of bias than RCTs, various approaches must be employed to assess bias risk. Authors do not always report all of the results of specific investigations. As a result, systematic review researchers should try to collect them directly from authors or through internet sources such as public websites or university-pharmaceutical company collaborations.
Considerations for Studying the Subject
In most circumstances, meta-analyses can only use aggregated data. The calculation of unadjusted intervention effects is possible in this case. Meta-analyses with adjusted intervention effects, where the adjusters are the same or substantially similar to those in the included studies, are the exceptions. The best-case scenario is to have IPD, which allows for further analyses such as trial and confounder correction, regression analyses, and subgroup analysis with adjustment. IPD meta-analyses should be planned ahead of time, with sponsorship permissions acquired in advance.
Editing More than 200,000 Words a Day
Send us Your Manuscript to Further Your Publication.







Is ChatGPT Trustworthy? | Rovedar | Scoop.it says:
ChatGPT vs. Human Editor | Rovedar | Scoop.it says:
Enhancing Your Assignments with ChatGPT | Roved... says: